
Chemistry, 02.10.2019 01:00 divagothboi
During an exothermic reaction, δh for the reactants was 890 kj/mol. which of the following statements is correct about the δh for the products?
a. it is less than 890 kj/mol because the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
b. it is less than 890 kj/mol because the amount of energy required to break bonds is greater than the amount of energy released in forming bonds.
c. it is greater than 890 kj/mol because the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
d. it is greater than 890 kj/mol because the amount of energy required to break bonds is greater than the amount of energy released in forming bonds.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 11:50
Achemist needs to prepare a buffer solution of ph 8.80. what molarity of nh3 (pkb = 4.75) is required to produce the buffer solution if the (nh4)2so4 in the solution is 1.8 m?
Answers: 1
You know the right answer?
During an exothermic reaction, δh for the reactants was 890 kj/mol. which of the following statement...
Questions

Mathematics, 18.09.2019 08:30

Mathematics, 18.09.2019 08:30

Mathematics, 18.09.2019 08:30




History, 18.09.2019 08:30

Physics, 18.09.2019 08:30



Mathematics, 18.09.2019 08:30

Health, 18.09.2019 08:30

Mathematics, 18.09.2019 08:30




Mathematics, 18.09.2019 08:30

Mathematics, 18.09.2019 08:30


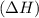 comes out to be positive.
comes out to be positive.


