
Chemistry, 08.10.2019 02:30 polyanskiymichael
Read the chemical equation. n2 + 3h2 → 2nh3 using the volume ratio, determine how many liters of nh3 is produced if 1.2 liters of h2 reacts with an excess of n2, if all measurements are taken at the same temperature and pressure? 1.8 liters 1.5 liters 0.90 liters 0.80 liters

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
Read the chemical equation. n2 + 3h2 → 2nh3 using the volume ratio, determine how many liters of nh3...
Questions

Mathematics, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00




Advanced Placement (AP), 23.01.2021 01:00

History, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00


English, 23.01.2021 01:00


English, 23.01.2021 01:00





Mathematics, 23.01.2021 01:00

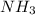 produced will be, 0.8 liters.
produced will be, 0.8 liters.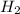 = 1.2 L
= 1.2 L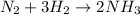
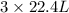 volume of
volume of 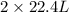 volume of
volume of 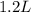 volume of
volume of 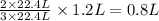 volume of
volume of 


