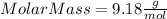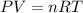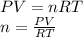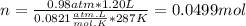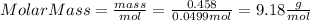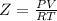Part 1. determine the molar mass of a 0.458-gram sample of gas having a volume of 1.20 l at 287 k and 0.980 atm. show your work. part 2. if this sample was placed under extreme pressure, describe how the actual volume would compare to the predicted volume. explain your answer.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1
You know the right answer?
Part 1. determine the molar mass of a 0.458-gram sample of gas having a volume of 1.20 l at 287 k an...
Questions

Mathematics, 05.02.2022 15:20


History, 05.02.2022 15:20

Mathematics, 05.02.2022 15:20


English, 05.02.2022 15:20

Biology, 05.02.2022 15:20


Mathematics, 05.02.2022 15:20


Mathematics, 05.02.2022 15:20





Mathematics, 05.02.2022 15:30



Mathematics, 05.02.2022 15:30

Biology, 05.02.2022 15:30