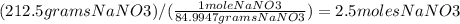Chemistry, 02.10.2019 08:50 itsjoke5550
Calculate the molarity of a solution in which 212.5g of nano3 are contained in 3.0 liters of solution.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
Calculate the molarity of a solution in which 212.5g of nano3 are contained in 3.0 liters of solutio...
Questions

Mathematics, 27.03.2021 09:30

Mathematics, 27.03.2021 09:30

English, 27.03.2021 09:30

Mathematics, 27.03.2021 09:30

Mathematics, 27.03.2021 09:30




History, 27.03.2021 09:30

Mathematics, 27.03.2021 09:30

History, 27.03.2021 09:30


Mathematics, 27.03.2021 09:30

Mathematics, 27.03.2021 09:30

English, 27.03.2021 09:30

Chemistry, 27.03.2021 09:30


Mathematics, 27.03.2021 09:30

Social Studies, 27.03.2021 09:30

Arts, 27.03.2021 09:40