
consider the substances below.
• 1 mol of beryllium
• 1 mol of salt
• 1 mol of water
• 1 mol of hydrogen
which statement is true about these substances? 1) they have exactly the same mass.2) they have different numbers of particles.3) they have the same number of atoms.4) they have different masses.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3


You know the right answer?
consider the substances below.
• 1 mol of beryllium
• 1 mol of salt
• 1 mol of water<...
• 1 mol of beryllium
• 1 mol of salt
• 1 mol of water<...
Questions


Mathematics, 14.01.2021 23:00


Mathematics, 14.01.2021 23:00

World Languages, 14.01.2021 23:00


Social Studies, 14.01.2021 23:00



History, 14.01.2021 23:00

Mathematics, 14.01.2021 23:00

Mathematics, 14.01.2021 23:00

Mathematics, 14.01.2021 23:00

English, 14.01.2021 23:00

English, 14.01.2021 23:00


Mathematics, 14.01.2021 23:00

Mathematics, 14.01.2021 23:00

Mathematics, 14.01.2021 23:00

Mathematics, 14.01.2021 23:00

 number of particles.
number of particles. number of atoms.
number of atoms. has 3 number of atoms. So, 1 mole of water contains
has 3 number of atoms. So, 1 mole of water contains 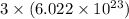 number of atoms.
number of atoms.

