
Chemistry, 22.01.2020 18:31 adrian1742
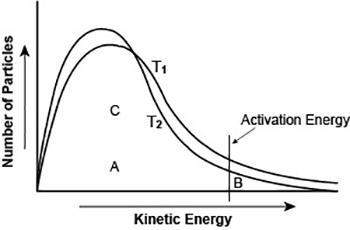
The graph shows the distribution of energy in the particles of two gas samples at different temperatures, t1 and t2. a, b, and c represent individual particles.
based on the graph, which of the following statements is likely to be true?
particle b is more likely to participate in the reaction than particle a.
particle c is more likely to participate in the reaction than particle b.
most of the gas particles have either very high or very low kinetic energies.
more gas particles participate in the reaction at t2 than at t1.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2


Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
The graph shows the distribution of energy in the particles of two gas samples at different temperat...
Questions


Mathematics, 30.11.2020 22:10


Arts, 30.11.2020 22:10

Mathematics, 30.11.2020 22:10


Spanish, 30.11.2020 22:10










Business, 30.11.2020 22:10

Geography, 30.11.2020 22:10

Mathematics, 30.11.2020 22:10



