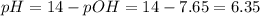Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

You know the right answer?
What is the ph of a solution with a 2.20 × 10−8 m hydroxide ion concentration?...
Questions

Geography, 21.11.2019 06:31

Mathematics, 21.11.2019 06:31

Mathematics, 21.11.2019 06:31

Biology, 21.11.2019 06:31

History, 21.11.2019 06:31

Mathematics, 21.11.2019 06:31

Computers and Technology, 21.11.2019 06:31



Geography, 21.11.2019 06:31


Chemistry, 21.11.2019 06:31

Biology, 21.11.2019 06:31

Mathematics, 21.11.2019 06:31


Health, 21.11.2019 06:31

Biology, 21.11.2019 06:31

French, 21.11.2019 06:31


![[OH^-]=2.20\times 10^{-8} M](/tpl/images/0483/8092/66252.png)
![pOH=-\log[OH^-]](/tpl/images/0483/8092/fe336.png)
![pOH=-\log[2.20\times 10^{-8} M]=7.65](/tpl/images/0483/8092/749e3.png)