Caco3(s) ∆→cao(s) + co2(g).
if 13.2 g of co2 was produced from the thermal decomposition of 40...


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Pauling and lewis questioned the extreme definitions of bonds. they wondered if bonds might be described somewhere in between the two extremes (covalent and ionic). on the basis of experimental data,pauling confirmed that bonds could be ionic, covalent, and for those, in between, exhibit a degree of ionic character. he theorized that the major factor was how strongly the atoms in the bond attracted the electrons. pauling called this factor - the tendency of an atom to attract electrons in a bond.
Answers: 2

Chemistry, 21.06.2019 15:00
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1
You know the right answer?
Questions


Mathematics, 29.08.2019 02:30


Mathematics, 29.08.2019 02:30

Computers and Technology, 29.08.2019 02:30

English, 29.08.2019 02:30

History, 29.08.2019 02:30



Chemistry, 29.08.2019 02:30


History, 29.08.2019 02:30

Mathematics, 29.08.2019 02:30


Health, 29.08.2019 02:30

Mathematics, 29.08.2019 02:30


Spanish, 29.08.2019 02:30


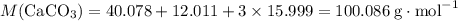 .
.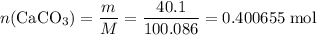 .
.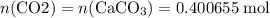 .
.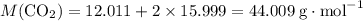 .
.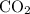 expected for the 40.1 grams of CaCO₃:
expected for the 40.1 grams of CaCO₃: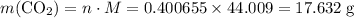 .
. .
.

