
Chemistry, 22.12.2019 12:31 icecreamisgood4u
One of the main components of pearls is calcium carbonate (caco3). if pearls are put into an acidic solution, they dissolve: caco3(s) + 2hcl(aq) ➡ cacl2(aq) + h2o(l) + co2(g). how many moles of caco3 can be dissolved in 0.0250 moles of hydrochloric acid?
0.0125 moles
0.0500 moles
2.50 moles
4.50 moles

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1


Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
One of the main components of pearls is calcium carbonate (caco3). if pearls are put into an acidic...
Questions

Biology, 24.07.2019 17:30

Social Studies, 24.07.2019 17:30

Social Studies, 24.07.2019 17:30

Biology, 24.07.2019 17:30

Physics, 24.07.2019 17:30





Computers and Technology, 24.07.2019 17:30




Business, 24.07.2019 17:30



Chemistry, 24.07.2019 17:30


Social Studies, 24.07.2019 17:30

![CaCO_3(s)+2HCl(aq)]\rightarrow CaCl_2(aq)+H_2O(l)+CO_2(g)](/tpl/images/0429/7730/2251f.png)
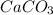
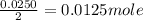 of
of 

