
Chemistry, 28.01.2020 13:40 heyyyyy3922
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o2) are carbon dioxide (co2) and water (h2o).
a mass of 15.51 g for an unknown fuel was combusted in a reaction vessel containing an unknown amount of oxygen. at the end of the reaction, there still remained 15.46 g of the fuel as well as 0.0817 g of water and 0.1497 g of carbon dioxide. the oxygen was completely consumed during the reaction.
how many molecules of oxygen gas were initially present in the reaction vessel?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1



Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o...
Questions


Mathematics, 04.02.2020 15:49

History, 04.02.2020 15:49



Physics, 04.02.2020 15:49

History, 04.02.2020 15:49

Physics, 04.02.2020 15:50

Mathematics, 04.02.2020 15:50

Geography, 04.02.2020 15:50

Business, 04.02.2020 15:50


Mathematics, 04.02.2020 15:50

Biology, 04.02.2020 15:50

Mathematics, 04.02.2020 15:50

History, 04.02.2020 15:50

Social Studies, 04.02.2020 15:50

Geography, 04.02.2020 15:50

Chemistry, 04.02.2020 15:50

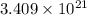
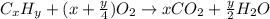


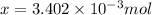
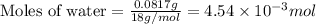
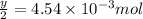
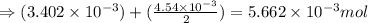
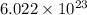 number of molecules.
number of molecules.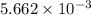 moles of oxygen will contain =
moles of oxygen will contain = 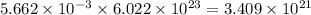 molecules.
molecules.

