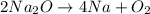Determine the atom inventory for the following equation: 2na2o ➡ 4na + o2. does this equation follow the law of conservation of mass?
na = 2, o = 1; na = 4, o = 1; no, this equation does not follow the law of conservation of mass
na = 4, o = 2; na = 4, o = 2; yes, this equation does follow the law of conservation of mass
na = 4, o = 4; na = 4, o = 2; yes, this equation does follow the law of conservation of mass
na = 2, o = 2; na = 4, o = 2; no, this equation does not follow the law of conservation of mass

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g
Answers: 2

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
Determine the atom inventory for the following equation: 2na2o ➡ 4na + o2. does this equation follo...
Questions


History, 25.01.2020 22:31

Computers and Technology, 25.01.2020 22:31



Chemistry, 25.01.2020 22:31



Mathematics, 25.01.2020 22:31


Computers and Technology, 25.01.2020 22:31

History, 25.01.2020 22:31


Business, 25.01.2020 22:31



History, 25.01.2020 22:31