
Chemistry, 23.09.2019 15:30 babyrocks7300
If 30.0 ml of ca(oh)2 with an unknown concentration is neutralized by 40.0 ml of 0.175 m hcl, what is the concentration of the ca(oh)2 solution? show all of the work needed to solve this problem. (2 points) ca(oh)2 + 2hcl yields 2h2 o + cacl2

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1

Chemistry, 23.06.2019 13:40
Match these items with their examples. 1. liquid solution milk 2. solid solution aluminum foil 3. compound soda 4. colloid steel 5. element salt
Answers: 1
You know the right answer?
If 30.0 ml of ca(oh)2 with an unknown concentration is neutralized by 40.0 ml of 0.175 m hcl, what i...
Questions

Social Studies, 28.09.2019 00:40


Business, 28.09.2019 00:40

History, 28.09.2019 00:40


English, 28.09.2019 00:40



Mathematics, 28.09.2019 00:40

Mathematics, 28.09.2019 00:40







Arts, 28.09.2019 00:40


English, 28.09.2019 00:40

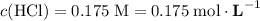 .
.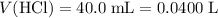 .
.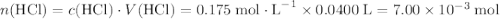 .
.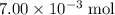 of HCl will neutralize only half that much Ca(OH)₂.
of HCl will neutralize only half that much Ca(OH)₂.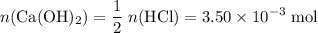 .
.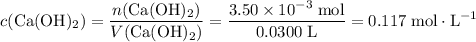 .
.

