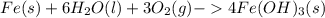One of the most recognizable corrosion reactions is the rusting of iron. rust is caused by iron reacting with oxygen gas in the presence of water to create an oxide layer. iron can form several different oxides, each having its own unique color. red rust is caused by the formation of iron(iii) oxide trihydrate. in the space provided, write the balanced reaction for the formation of fe2o3•3h2o(s). phases are optional.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
One of the most recognizable corrosion reactions is the rusting of iron. rust is caused by iron reac...
Questions


Mathematics, 14.07.2020 02:01



Mathematics, 14.07.2020 02:01


Mathematics, 14.07.2020 02:01




Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Arts, 14.07.2020 02:01

Health, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01