
One of the main components of an airbag is the gas that fills it. as part of the design process, you need to determine the exact amount of nitrogen that should be produced. calculate the number of moles of nitrogen required to fill the airbag. show your work. assume that the nitrogen produced by the chemical reaction is at a temperature of 495°c and that nitrogen gas behaves like an ideal gas. use this fact sheet to review the ideal gas law.

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
One of the main components of an airbag is the gas that fills it. as part of the design process, you...
Questions


Mathematics, 04.10.2021 01:10

Chemistry, 04.10.2021 01:10

Mathematics, 04.10.2021 01:10



English, 04.10.2021 01:10

Mathematics, 04.10.2021 01:10


English, 04.10.2021 01:20

Chemistry, 04.10.2021 01:20

Physics, 04.10.2021 01:20

Biology, 04.10.2021 01:20


Mathematics, 04.10.2021 01:20

World Languages, 04.10.2021 01:20

English, 04.10.2021 01:20


Mathematics, 04.10.2021 01:20

English, 04.10.2021 01:20

 ,
, 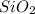 and
and 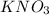 .The temperature at collision increases and
.The temperature at collision increases and 

