

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
The reaction a + 2b + c -- > d + 2e is first order in reactant a, first order in b, and second or...
Questions



Mathematics, 31.05.2021 19:00




Business, 31.05.2021 19:00

English, 31.05.2021 19:00


Computers and Technology, 31.05.2021 19:00

Geography, 31.05.2021 19:00

Physics, 31.05.2021 19:00


Mathematics, 31.05.2021 19:00

History, 31.05.2021 19:00

Mathematics, 31.05.2021 19:00

Business, 31.05.2021 19:00


History, 31.05.2021 19:00

![\text{Rate}=k[A][B][C]^2](/tpl/images/0287/3858/2c5a0.png)
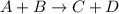
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0287/3858/10aeb.png)
![[A]](/tpl/images/0287/3858/6aa06.png) and
and ![[B]](/tpl/images/0287/3858/db909.png) = concentration of A and B reactant
= concentration of A and B reactant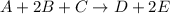
![\text{Rate}=k[A]^1[B]^1[C]^2](/tpl/images/0287/3858/d33f5.png)



