
Chemistry, 24.09.2019 03:50 absoul1143
For a gas to be soluble in water, the gas should contain (nonpolar, polar ) molecules. the water should be at a (high, low) temperature, and the gas should be at (high, low ) pressure above the surface of the water.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
For a gas to be soluble in water, the gas should contain (nonpolar, polar ) molecules. the water sho...
Questions

Mathematics, 13.02.2021 08:00

Chemistry, 13.02.2021 08:00


Biology, 13.02.2021 08:00

Social Studies, 13.02.2021 08:00

English, 13.02.2021 08:00


Mathematics, 13.02.2021 08:00

Mathematics, 13.02.2021 08:00









Mathematics, 13.02.2021 08:00


Mathematics, 13.02.2021 08:00

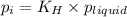
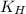 = Henry's constant =
= Henry's constant = 
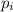 = partial pressure a gas
= partial pressure a gas 

