
Chemistry, 03.02.2020 14:01 benoitjaylewe
Before the reaction occurs, the concentration of a is 0.071m. if the concentration of a at equilibrium is 0.03195m, what is the equilibrium constant? a(g) = 2b(g) + c(g)
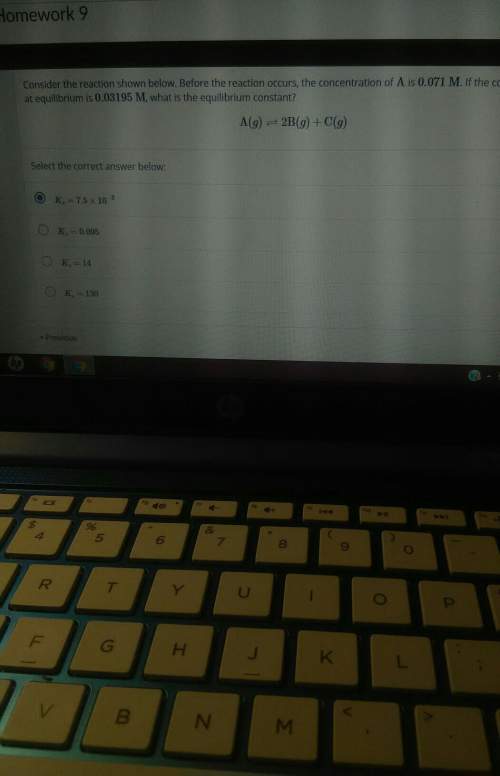

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
Before the reaction occurs, the concentration of a is 0.071m. if the concentration of a at equilibri...
Questions

Mathematics, 09.03.2021 20:00


Spanish, 09.03.2021 20:00

Mathematics, 09.03.2021 20:00







Mathematics, 09.03.2021 20:00


Biology, 09.03.2021 20:00




Mathematics, 09.03.2021 20:00

Mathematics, 09.03.2021 20:00

Mathematics, 09.03.2021 20:00

![[\text{B}]^{2}](/tpl/images/0496/7386/eabb8.png) . The coefficient in front of C is 1. Raise the concentration of C to the first power, which is the same as
. The coefficient in front of C is 1. Raise the concentration of C to the first power, which is the same as ![[\text{C}]](/tpl/images/0496/7386/9fa27.png) .
.![[\text{B}]^{2} \cdot[\text{C}]](/tpl/images/0496/7386/4f968.png) .
.![[\text{A}]](/tpl/images/0496/7386/b5122.png) .
.![\displaystyle K_c = \frac{[\text{B}\;(g)]^{2} \cdot[\text{C}\;(g)]}{[\text{A}\;(g)]}](/tpl/images/0496/7386/f0385.png) ,
,![[\text{B}]](/tpl/images/0496/7386/2080e.png) , and
, and 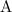 be
be 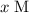 .
. 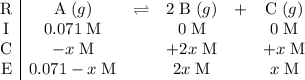 .
.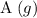 is
is 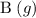 shall be
shall be 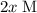 .
.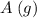 is
is 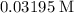 .
. 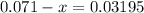 ,
,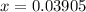 .
.![[\text{A}\;(g)] = 0.03195\;\text{M}](/tpl/images/0496/7386/682c0.png) according to the question;
according to the question;![[\text{B}\;(g)] = 2 x \;\text{M}= 0.0781\;\text{M}](/tpl/images/0496/7386/b95c9.png) as in the RICE table;
as in the RICE table;![[\text{C}\;(g)] = x = 0.03905\;\text{M}](/tpl/images/0496/7386/f31c2.png) as in the RICE table.
as in the RICE table.![\displaystyle \begin{aligned}K_c &= \frac{[\text{B}\;(g)]^{2} \cdot[\text{C}\;(g)]}{[\text{A}\;(g)]}\\&=\frac{{0.0781}^{2} \times 0.03905}{0.03195}\\&=7.5\times 10^{3}\;\text{M}\end{aligned}](/tpl/images/0496/7386/4e6aa.png) .
.

