
Chemistry, 04.02.2020 20:45 tyliyahmiles99
Calculate delta h in kj for the following reactions using heats of formation:
a) 2c2h6 (g) + 7o2 (g) > 4co2 (g) +6h2o (g)
b) 2pbo (s) + pbo2 (s) > pb3o4 (s)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Calculate delta h in kj for the following reactions using heats of formation:
a) 2c2h6 (g) +...
a) 2c2h6 (g) +...
Questions

Chemistry, 10.04.2021 22:20

Social Studies, 10.04.2021 22:20




Mathematics, 10.04.2021 22:20

Mathematics, 10.04.2021 22:20

Mathematics, 10.04.2021 22:20

English, 10.04.2021 22:20

Mathematics, 10.04.2021 22:20

Mathematics, 10.04.2021 22:20

Mathematics, 10.04.2021 22:20



Health, 10.04.2021 22:30





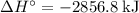 per mole reaction.
per mole reaction. per mole reaction.
per mole reaction.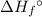 of a substance?
of a substance? 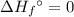 for the most stable allotrope of each element under standard conditions. For example, oxygen
for the most stable allotrope of each element under standard conditions. For example, oxygen 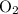 (not ozone
(not ozone 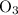 ) is the most stable allotrope of oxygen. Also, under STP
) is the most stable allotrope of oxygen. Also, under STP 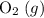 from itself does not involve any chemical or physical change. As a result,
from itself does not involve any chemical or physical change. As a result, 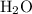 in particular) and the sign of the enthalpy changes.
in particular) and the sign of the enthalpy changes.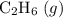 :
: 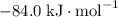 ;
;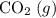 :
: 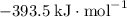 ;
;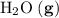 :
: 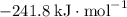 ;
;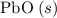 :
: 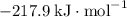 ;
;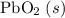 :
: 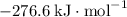 ;
;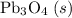 :
: 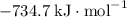
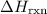 (or simply
(or simply 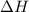 from enthalpies of formation?
from enthalpies of formation?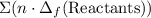 to show that this value takes the coefficients into account.Multiply the enthalpy of formation of each reactant by its coefficient in the equation.Find the sum of these values. Label the sum
to show that this value takes the coefficients into account.Multiply the enthalpy of formation of each reactant by its coefficient in the equation.Find the sum of these values. Label the sum 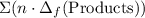 to show that this value takes the coefficient into account.Change = Final - Initial. So is the case with enthalpy changes.
to show that this value takes the coefficient into account.Change = Final - Initial. So is the case with enthalpy changes. 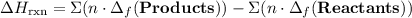 .
. 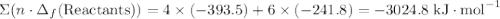 ;
;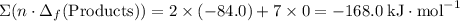 ;
;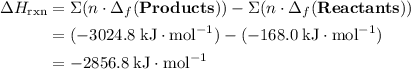 .
.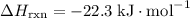 .
.

