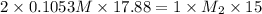Chemistry, 25.11.2019 23:31 josephsky420
A15.00-ml sample of a naoh solution of unknown concentration requires 17.88 ml of a 0.1053 m h2so4 solution to reach the equivalence point in a titration. what is the concentration of the naoh solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3


Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

You know the right answer?
A15.00-ml sample of a naoh solution of unknown concentration requires 17.88 ml of a 0.1053 m h2so4 s...
Questions





Biology, 27.02.2020 02:03

Mathematics, 27.02.2020 02:03



Mathematics, 27.02.2020 02:03


Mathematics, 27.02.2020 02:03

Mathematics, 27.02.2020 02:03




Chemistry, 27.02.2020 02:03




History, 27.02.2020 02:03

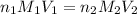
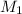 = molarity of
= molarity of 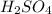 solution = 0.1053 M
solution = 0.1053 M
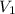 = volume of
= volume of 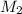 = molarity of
= molarity of 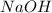 solution = 0.46 M
solution = 0.46 M
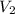 = volume of
= volume of 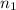 = valency of
= valency of 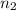 = valency of
= valency of