Chemistry, 03.02.2020 01:55 farhansayeed11
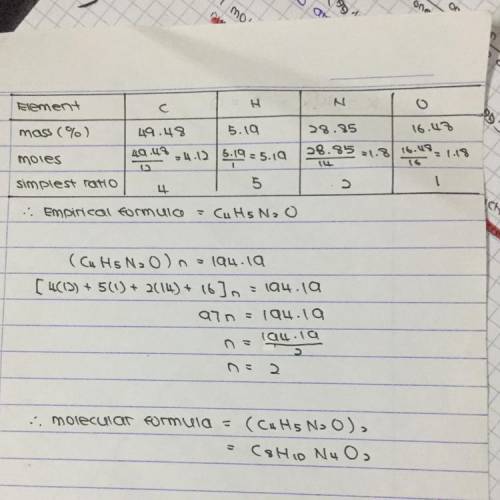
Caffeine has the following percent composition: carbon 49.48%, hydrogen 5.19%, oxygen 16.48% and nitrogen 28.85%. its molecular weight is 194.19 g/mol. what is its molecular formula?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1


Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
Caffeine has the following percent composition: carbon 49.48%, hydrogen 5.19%, oxygen 16.48% and ni...
Questions

Mathematics, 12.11.2020 21:30

English, 12.11.2020 21:30


Biology, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30


English, 12.11.2020 21:30

History, 12.11.2020 21:30





Social Studies, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30