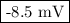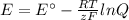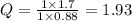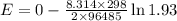Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Strong conductivity of plasma allows it to act and react as and
Answers: 2


Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1
You know the right answer?
What would the potential of a standard hydrogen electrode (s. h.e.) be if it was under the following...
Questions

Mathematics, 13.01.2020 13:31

Mathematics, 13.01.2020 13:31

History, 13.01.2020 13:31

Mathematics, 13.01.2020 13:31

Social Studies, 13.01.2020 13:31






Mathematics, 13.01.2020 13:31


Mathematics, 13.01.2020 13:31





Mathematics, 13.01.2020 13:31


Arts, 13.01.2020 13:31