
Chemistry, 21.01.2020 02:31 marquezsturgis
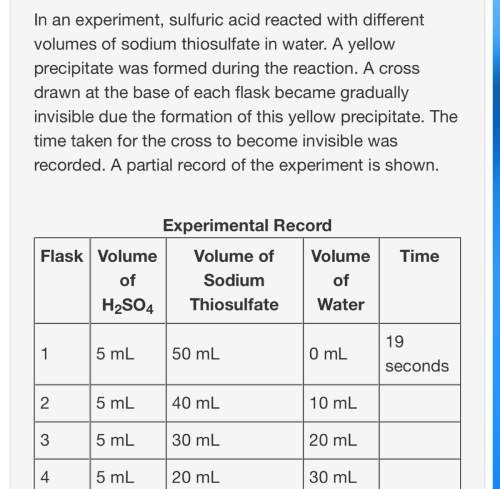
Based on your knowledge of factors that affect the rates of chemical reactions, predict the trend in the last column of the experimental record. use complete sentences to explain the trend you predicted. you do not have to determine exact values for time; just describe the trend you would expect (increase or decrease) and why it occurs
- answer asap! will award brainlist

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 11:30
Which of the following is a property of nonmetals? a.nonmetals are ductile. b.nonmetals have a shiny luster. c.nonmetals have high density. d.nonmetals are nonconductors.
Answers: 1
You know the right answer?
Based on your knowledge of factors that affect the rates of chemical reactions, predict the trend in...
Questions

Chemistry, 05.02.2021 14:00

Mathematics, 05.02.2021 14:00





Mathematics, 05.02.2021 14:00

Mathematics, 05.02.2021 14:00

Mathematics, 05.02.2021 14:00




Mathematics, 05.02.2021 14:00

Biology, 05.02.2021 14:00


Mathematics, 05.02.2021 14:00


Mathematics, 05.02.2021 14:00

Computers and Technology, 05.02.2021 14:00



