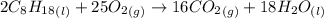Chemistry, 26.08.2019 21:00 ilovebeans25423
The combustion of octane, c8h18, proceeds according to the reaction
2c8h18(l)+25o2(> 16co2(g)+18h2o(l)
if 297 mol of octane combusts, what volume of carbon dioxide is produced at 20.0 °c and 0.995 atm?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

You know the right answer?
The combustion of octane, c8h18, proceeds according to the reaction
2c8h18(l)+25o2(> 16co2(...
2c8h18(l)+25o2(> 16co2(...
Questions

Mathematics, 12.12.2020 16:20

English, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

History, 12.12.2020 16:20

Health, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20



Chemistry, 12.12.2020 16:20

Biology, 12.12.2020 16:20





Chemistry, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20



Mathematics, 12.12.2020 16:20