Chemistry, 31.01.2020 13:56 sierravick123owr441
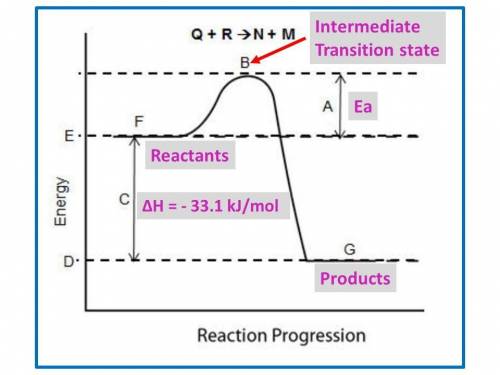
B. for the following questions, use the reaction no2(g) n2(g) + o2(g), with δh = –33.1 kj/mol and δs= 63.02 j/(mol·k).
i. draw a possible potential energy diagram of the reaction. label the enthalpy of the reaction.
ii. is the reaction endothermic or exothermic? explain your answer. (2 points)
iii. what is the gibbs free energy of the reaction at 25°c?
iv. is the reaction spontaneous or nonspontaneous at 25°c? explain your answer.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1
You know the right answer?
B. for the following questions, use the reaction no2(g) n2(g) + o2(g), with δh = –33.1 kj/mol and δs...
Questions




Social Studies, 19.02.2022 15:50







Chemistry, 19.02.2022 16:00


History, 19.02.2022 16:00

Mathematics, 19.02.2022 16:00



Mathematics, 19.02.2022 16:00


Advanced Placement (AP), 19.02.2022 16:10