
Chemistry, 02.10.2019 22:40 smarty5187
From the data below, calculate the total heat (in j) needed to convert 0.782 mol of gaseous ethanol at 300.0°c and 1 atm to liquid ethanol at 25.0°c and 1 atm

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
From the data below, calculate the total heat (in j) needed to convert 0.782 mol of gaseous ethanol...
Questions

Mathematics, 20.01.2020 07:31


Mathematics, 20.01.2020 07:31


Biology, 20.01.2020 07:31

English, 20.01.2020 07:31

Chemistry, 20.01.2020 07:31

History, 20.01.2020 07:31



Spanish, 20.01.2020 07:31








Mathematics, 20.01.2020 07:31

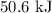 .
.

