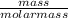Chemistry, 02.11.2019 23:31 pgjohnston001
A55.0g sample of iron (iii) filings is reacted with 23.8g of powdered sulfur (s8). how much iron (iii) sulfide in moles would be produced in this reaction?
equation:
convert to moles
of iron:
convert to moles
of sulfur:
calculate the
limiting reagent:
solve the problem:

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
A55.0g sample of iron (iii) filings is reacted with 23.8g of powdered sulfur (s8). how much iron (ii...
Questions

Mathematics, 18.10.2020 06:01


English, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

History, 18.10.2020 06:01

History, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

Medicine, 18.10.2020 06:01




Mathematics, 18.10.2020 06:01



Mathematics, 18.10.2020 06:01




Social Studies, 18.10.2020 06:01