
Chemistry, 03.02.2020 01:52 alanis337455p4xzek
When solutions of silver nitrate, agno3, and calcium iodide, cai2, are mixed, a yellow precipitate of silver iodide is formed. calculate the mass of silver iodide that is formed if 50.00 ml of 1.00 m agno3 is combined with 30.00 ml of 1.25 m cai2.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
When solutions of silver nitrate, agno3, and calcium iodide, cai2, are mixed, a yellow precipitate o...
Questions

Health, 29.01.2021 20:50


Arts, 29.01.2021 20:50




Mathematics, 29.01.2021 20:50

History, 29.01.2021 20:50


Mathematics, 29.01.2021 20:50



Mathematics, 29.01.2021 20:50

Health, 29.01.2021 20:50

Health, 29.01.2021 20:50

Mathematics, 29.01.2021 20:50

Mathematics, 29.01.2021 20:50

Mathematics, 29.01.2021 20:50

Mathematics, 29.01.2021 20:50

History, 29.01.2021 20:50

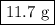
 .
.


