Chemistry, 30.01.2020 01:48 evanwall91
Linda performed the following trials in an experiment. trial 1: heat 30.0 grams of water at 0 °c to a final temperature of 40.0 °c. trial 2: heat 40.0 grams of water at 30.0 °c to a final temperature of 40.0 °c. which statement is true about the experiments?
(a) the heat absorbed in trial 1 is about 1,674 j greater than the heat absorbed in trial 2.
(b)the heat absorbed in trial 1 is about 3,347 j greater than the heat absorbed in trial 2.
(c)the same amount of heat is absorbed in both the experiments because the heat absorbed depends only on the final temperature.
(c) the same amount of heat is absorbed in both the experiments because the product of mass, specific heat capacity, and change in temperature are the same.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Linda performed the following trials in an experiment. trial 1: heat 30.0 grams of water at 0 °c to...
Questions

Spanish, 18.09.2019 10:30

Arts, 18.09.2019 10:30


Physics, 18.09.2019 10:30

Mathematics, 18.09.2019 10:30



Social Studies, 18.09.2019 10:30



Business, 18.09.2019 10:30



Mathematics, 18.09.2019 10:30


Mathematics, 18.09.2019 10:30


History, 18.09.2019 10:30

English, 18.09.2019 10:30

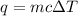 .....(1)
.....(1) = Change in temperature
= Change in temperature![m=30g\\\Delta T=[40-0]^oC=40^oC\\q=?J](/tpl/images/0484/0461/0fc16.png)
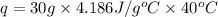
![m=40g\\\Delta T=[40-30]^oC=10^oC\\q=?J](/tpl/images/0484/0461/51775.png)