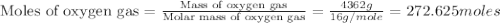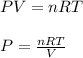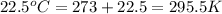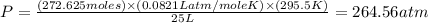Chemistry, 22.09.2019 19:20 glocurlsprinces
Using the ideal gas equation, calculate the pressure of oxygen gas in a cylinder with a volume of 25.00 l. the oxygen masses 4.362 kg and room temperature is at 22.5 o c. how many moles of oxygen are there and what is the pressure of oxygen in atmospheres in the cylinder according to the ideal gas law?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
Using the ideal gas equation, calculate the pressure of oxygen gas in a cylinder with a volume of 25...
Questions

History, 17.10.2019 16:00

Social Studies, 17.10.2019 16:00


English, 17.10.2019 16:00





Mathematics, 17.10.2019 16:00

English, 17.10.2019 16:00

English, 17.10.2019 16:00

Mathematics, 17.10.2019 16:00


Mathematics, 17.10.2019 16:00


Advanced Placement (AP), 17.10.2019 16:00




English, 17.10.2019 16:00