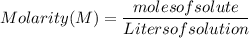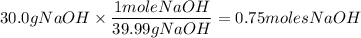Chemistry, 25.09.2019 16:30 ariellencevallo
What is the molarity of a solution prepared by dissolving 30.0 grams of naoh in enough water to make a solution with a total volume of 2.40 liters?
0.150 m naoh
0.218 m naoh
0.313 m naoh
0.462 m naoh

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
What is the molarity of a solution prepared by dissolving 30.0 grams of naoh in enough water to make...
Questions


Mathematics, 29.08.2019 13:00



History, 29.08.2019 13:00





Mathematics, 29.08.2019 13:00

World Languages, 29.08.2019 13:00





English, 29.08.2019 13:00


Social Studies, 29.08.2019 13:00


History, 29.08.2019 13:00