
Chemistry, 23.11.2019 19:31 millerbe1228
Determine the enthalpy:
4nh3(g) + 7o2(g) −→ 4no2(g) + 6h2o(ℓ)
∆h◦ f species ( kj mol)
nh3(g) −46.11
no2(g) +33.2
h2o(ℓ) −285.83
1. −1899 kj/mol rxn
2. +2034.4 kj/mol rxn
3. −298.7 kj/mol rxn
4. −1766 kj/mol rxn
5. −1397.6 kj/mol rxn

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
Determine the enthalpy:
4nh3(g) + 7o2(g) −→ 4no2(g) + 6h2o(ℓ)
∆h◦ f species ( kj...
4nh3(g) + 7o2(g) −→ 4no2(g) + 6h2o(ℓ)
∆h◦ f species ( kj...
Questions

Mathematics, 28.01.2021 18:40



Mathematics, 28.01.2021 18:40

History, 28.01.2021 18:40


English, 28.01.2021 18:40

Mathematics, 28.01.2021 18:40

English, 28.01.2021 18:40

English, 28.01.2021 18:40

Mathematics, 28.01.2021 18:40


Mathematics, 28.01.2021 18:40





Social Studies, 28.01.2021 18:40

History, 28.01.2021 18:40

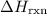 from the standard enthalpy of formation
from the standard enthalpy of formation 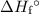 of each species.
of each species.![\Sigma \left[\Delta H_f\textdegree{}(\text{Products})]\right](/tpl/images/0388/1909/1223d.png) . Multiply the
. Multiply the ![\Sigma \left[\Delta H_f\textdegree{}(\text{Reactants})]\right](/tpl/images/0388/1909/51ea9.png) . Similarly, multiply the
. Similarly, multiply the 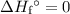 for the most stable allotrope of each elements under their standard state. For example,
for the most stable allotrope of each elements under their standard state. For example, 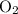 (not
(not 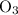 ozone) is the most stable allotrope of oxygen under STP.
ozone) is the most stable allotrope of oxygen under STP. 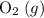 . Note the gaseous state symbol. This value is implied such that it's not provided in the question.
. Note the gaseous state symbol. This value is implied such that it's not provided in the question. ![\Sigma \left[\Delta H_f\textdegree{}(\textbf{Products})]\right = 4 \times (+33.2) + 6\times (-285.83) = -1582.18\;\text{kJ}\cdot\text{mol}^{-1}](/tpl/images/0388/1909/4e1c4.png) .
.![\Sigma \left[\Delta H_f\textdegree{}(\textbf{Reactants})]\right = 4 \times (-46.11) + 7\times 0 = -184.44 \;\text{kJ}\cdot\text{mol}^{-1}](/tpl/images/0388/1909/4a036.png) .
.![\begin{aligned} \Delta H_\text{rxn} &= \Sigma \left[\Delta H_f\textdegree{}(\textbf{Products})]\right - \Sigma \left[\Delta H_f\textdegree{}(\textbf{Reactants})]\right\\ &= -1582.18\;\text{kJ}\cdot\text{mol}^{-1} - (-184.44 \;\text{kJ}\cdot\text{mol}^{-1})\\ &= -1397.74\;\text{kJ}\cdot\text{mol}^{-1}\end{aligned}](/tpl/images/0388/1909/dcaf7.png) .
.

