
Chemistry, 06.12.2019 05:31 taylortayshaun7
The radioactive decay of a certain sample produced 846 disintegrations per minute. exactly 3.00 days later, the rate of decay was found to be 269 disintegrations per minute. calculate the half-life, in days, for the decay of this sample.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
The radioactive decay of a certain sample produced 846 disintegrations per minute. exactly 3.00 days...
Questions

Arts, 14.12.2020 23:00


Mathematics, 14.12.2020 23:00

Mathematics, 14.12.2020 23:00


Geography, 14.12.2020 23:00


Mathematics, 14.12.2020 23:00

Mathematics, 14.12.2020 23:00



Mathematics, 14.12.2020 23:00

Mathematics, 14.12.2020 23:00



Mathematics, 14.12.2020 23:00

History, 14.12.2020 23:00



Biology, 14.12.2020 23:00

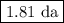
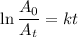
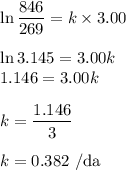
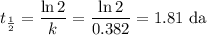
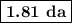 .
.

