
Chemistry, 08.10.2019 18:40 justin20080
The specific heat of a certain type of cooking oil is 1.75 j/(g⋅°c). how much heat energy is needed to raise the temperature of 2.94 kg of this oil from 23 °c to 191 °c?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
The specific heat of a certain type of cooking oil is 1.75 j/(g⋅°c). how much heat energy is needed...
Questions







Mathematics, 19.02.2021 01:50




Mathematics, 19.02.2021 01:50

Mathematics, 19.02.2021 01:50

Mathematics, 19.02.2021 01:50



Physics, 19.02.2021 01:50




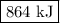
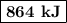 .
.



