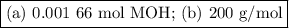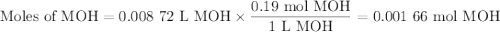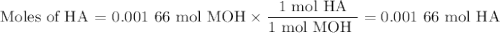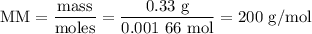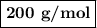Chemistry, 20.10.2019 06:50 goldenarrow
Using the concentration of the base and the volume of the base used, calculate the moles of the base used in the titration. then, using the mass of the acid, determine the molar mass of the acid.
data:
concentration of the base(naoh)= 0.19 m
volume of the base used= 8.72 ml
mass of the acid(unknown)= 0.33 g

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2
You know the right answer?
Using the concentration of the base and the volume of the base used, calculate the moles of the base...
Questions

English, 25.06.2019 09:30





Mathematics, 25.06.2019 09:30




Mathematics, 25.06.2019 09:30



History, 25.06.2019 09:30







Mathematics, 25.06.2019 09:30