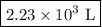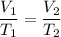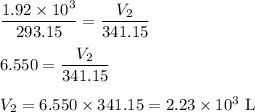Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Asample of gas in a balloon has an initial temperature of 20. ∘c and a volume of 1.92×103 l . if the...
Questions



Social Studies, 13.02.2020 21:03

Mathematics, 13.02.2020 21:03

Mathematics, 13.02.2020 21:03


English, 13.02.2020 21:03

History, 13.02.2020 21:03



History, 13.02.2020 21:03

Mathematics, 13.02.2020 21:03







Chemistry, 13.02.2020 21:03