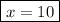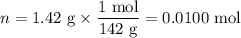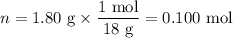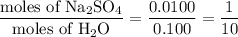Chemistry, 20.01.2020 08:31 mercedesamatap21hx0
When a 3.22 g sample of an unknown hydrate of sodium sulfate, na2so4 . x h2o (s), is heated, h2o (molar mass 18 g) is driven off. the mass of the anhydrous na2so4 (s) (molar mass 142 g) that remains is 1.42g. the value of x in the hydrate is

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
When a 3.22 g sample of an unknown hydrate of sodium sulfate, na2so4 . x h2o (s), is heated, h2o (mo...
Questions


Mathematics, 05.07.2019 14:30

Mathematics, 05.07.2019 14:30





Mathematics, 05.07.2019 14:30

History, 05.07.2019 14:30

Social Studies, 05.07.2019 14:30





Arts, 05.07.2019 14:30


Mathematics, 05.07.2019 14:30

Geography, 05.07.2019 14:30

Social Studies, 05.07.2019 14:30

Mathematics, 05.07.2019 14:30