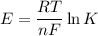Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1
You know the right answer?
From standard reduction potentials, calculate the equilibrium constant at 25 ∘c for the reaction 2mn...
Questions

Biology, 11.10.2019 05:30

English, 11.10.2019 05:30


Biology, 11.10.2019 05:30



Physics, 11.10.2019 05:30

Mathematics, 11.10.2019 05:30

History, 11.10.2019 05:30

Advanced Placement (AP), 11.10.2019 05:30

Mathematics, 11.10.2019 05:30







History, 11.10.2019 05:30

English, 11.10.2019 05:30