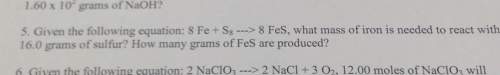Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1
You know the right answer?
5. given the following equation: 8 fe s8 8 fes, what mass of iron is needed to react with16.0 grams...
Questions

Mathematics, 27.02.2020 00:12



Mathematics, 27.02.2020 00:12




Mathematics, 27.02.2020 00:12



History, 27.02.2020 00:12

Mathematics, 27.02.2020 00:12







Chemistry, 27.02.2020 00:13

History, 27.02.2020 00:13