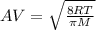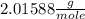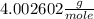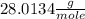Chemistry, 24.01.2020 03:31 crystaldewar55C
At 298 k the average kinetic energy is the same for h2, he, and n2. the gas with the highest average velocity is: a) h2b) hec) n2d) all have the same average velocity

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
At 298 k the average kinetic energy is the same for h2, he, and n2. the gas with the highest average...
Questions


Chemistry, 18.01.2020 03:31


Mathematics, 18.01.2020 03:31

Mathematics, 18.01.2020 03:31






English, 18.01.2020 03:31





Mathematics, 18.01.2020 03:31

Physics, 18.01.2020 03:31