
Chemistry, 18.11.2019 11:31 briarwilliams9668
Need asap
the mass of cobalt-60 in a sample decreased from 0.800g to 0.200g over a period of 10.5 years. from this information, calculate the half-life of cobalt-60.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1
You know the right answer?
Need asap
the mass of cobalt-60 in a sample decreased from 0.800g to 0.200g over a pe...
the mass of cobalt-60 in a sample decreased from 0.800g to 0.200g over a pe...
Questions





Spanish, 16.12.2020 23:10



Advanced Placement (AP), 16.12.2020 23:10



Mathematics, 16.12.2020 23:10



Biology, 16.12.2020 23:10

English, 16.12.2020 23:10





Mathematics, 16.12.2020 23:10

 .
.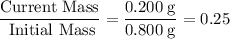 .
.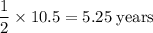 .
.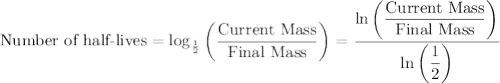 .
.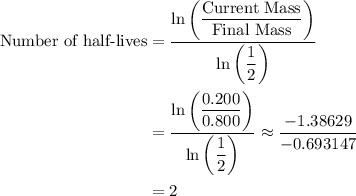 .
.

