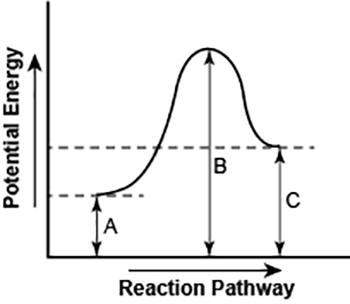
the diagram shows the potential energy changes for a reaction pathway.

Question:
the diagram shows the potential energy changes for a reaction pathway.
part 1: does the diagram illustrate an endothermic or an exothermic reaction? give reasons in support of your answer.
part 2: describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.
part 1: the diagram illustrates an endothermic reaction as the products has a higher potential energy than the reactants do. there is a positive slope of the diagram and there is enough energy to meet the activation energy requirement.
part 2: you can determine the total change in enthalpy and activation energy from the diagram by the potential energy of the reactants. if the reactants have a high potential energy, then the enthalpy is also high, and if the reactants have a low potential energy, then the enthalpy is low. you can determine if the diagram is positive or negative by knowing if its an endothermic or exothermic reaction. an endothermic reaction is positive because the products are higher than the reactants and a exothermic reaction is negative because the reactants are higher than the products.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
Question:
the diagram shows the potential energy changes for a reaction pathway.
the diagram shows the potential energy changes for a reaction pathway.
Questions

Geography, 26.08.2019 21:30

Mathematics, 26.08.2019 21:30



History, 26.08.2019 21:30


Social Studies, 26.08.2019 21:30

History, 26.08.2019 21:30


Social Studies, 26.08.2019 21:30

Social Studies, 26.08.2019 21:30

Mathematics, 26.08.2019 21:30



Spanish, 26.08.2019 21:30


Mathematics, 26.08.2019 21:30

Geography, 26.08.2019 21:30


Health, 26.08.2019 21:30



