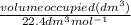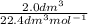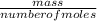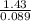Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2


Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1

Chemistry, 23.06.2019 19:00
How can evidence from an experiment be explained in her relationship to the hypothesis? a.as a prediction b.as a question c.as an in inference d.as a conclusion
Answers: 2
You know the right answer?
Asample of gas with a volume of 2.0 l at stp is found to have a mass of 1.43 g. calculate the molecu...
Questions


Physics, 25.02.2021 23:10



History, 25.02.2021 23:10

Spanish, 25.02.2021 23:10



English, 25.02.2021 23:10

Mathematics, 25.02.2021 23:10

Spanish, 25.02.2021 23:10

Mathematics, 25.02.2021 23:10


Mathematics, 25.02.2021 23:10





Mathematics, 25.02.2021 23:10

Social Studies, 25.02.2021 23:10