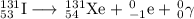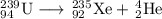Some radioactive nuclides have very short half-lives, for example, i-31 has a half-life of approximately 8 days. pu-234, by comparison has a half-life of 24,000 years. explain why both of these examples are dangerous, even though their half-lives are very different. be sure to describe the different major types of radiation, and their hazards. (radioactive decay and half-life)

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

You know the right answer?
Some radioactive nuclides have very short half-lives, for example, i-31 has a half-life of approxima...
Questions

Social Studies, 08.02.2021 17:40


Mathematics, 08.02.2021 17:40

History, 08.02.2021 17:40

Mathematics, 08.02.2021 17:40

Mathematics, 08.02.2021 17:40

Mathematics, 08.02.2021 17:40

English, 08.02.2021 17:40



History, 08.02.2021 17:40

Mathematics, 08.02.2021 17:40


Mathematics, 08.02.2021 17:40

Mathematics, 08.02.2021 17:40

Mathematics, 08.02.2021 17:40

Mathematics, 08.02.2021 17:40


History, 08.02.2021 17:40

Biology, 08.02.2021 17:40