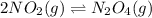Chemistry, 29.06.2019 21:50 dooboose15
In the reversible reaction: 2no2 (g) ⇌ n2o4 (g) the formation of dinitrogen tetroxide releases heat and the formation of nitrogen dioxide absorbs heat. if the reaction is at equilibrium and the temperature increases, what will the effect be? a. nitrogen dioxide absorbs heat and changes from gas to liquid. b. the equilibrium will shift so that there is more nitrogen dioxide. c. the equilibrium will shift so that there is more dinitrogen tetroxide. d. dinitrogen tetroxide absorbs heat and changes from gas to liquid.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
In the reversible reaction: 2no2 (g) ⇌ n2o4 (g) the formation of dinitrogen tetroxide releases heat...
Questions


Geography, 02.11.2020 19:00

Mathematics, 02.11.2020 19:00

History, 02.11.2020 19:00

Arts, 02.11.2020 19:00

Chemistry, 02.11.2020 19:00




Mathematics, 02.11.2020 19:00


Mathematics, 02.11.2020 19:00




Mathematics, 02.11.2020 19:00

Arts, 02.11.2020 19:00


Biology, 02.11.2020 19:00