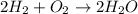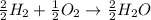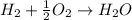Chemistry, 29.06.2019 11:20 dakotaadkins1818
Will mark ! read the chemical equation. 2h2 + o2 → 2h2o which of the following statements would be correct if one mole of hydrogen was used in this reaction? a. one mole of oxygen was used in this reaction. b. two moles of oxygen were used in this reaction. c. one mole of water was produced from this reaction. d. two moles of water were produced from this reaction.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
Will mark ! read the chemical equation. 2h2 + o2 → 2h2o which of the following statements would be...
Questions


Computers and Technology, 27.10.2019 09:43

Mathematics, 27.10.2019 09:43

Mathematics, 27.10.2019 09:43

Mathematics, 27.10.2019 09:43

Biology, 27.10.2019 09:43

Arts, 27.10.2019 09:43



Arts, 27.10.2019 09:43


Mathematics, 27.10.2019 09:43



Social Studies, 27.10.2019 09:43

Biology, 27.10.2019 09:43

Geography, 27.10.2019 09:43



English, 27.10.2019 09:43