
Chemistry, 29.06.2019 08:20 davelopez979
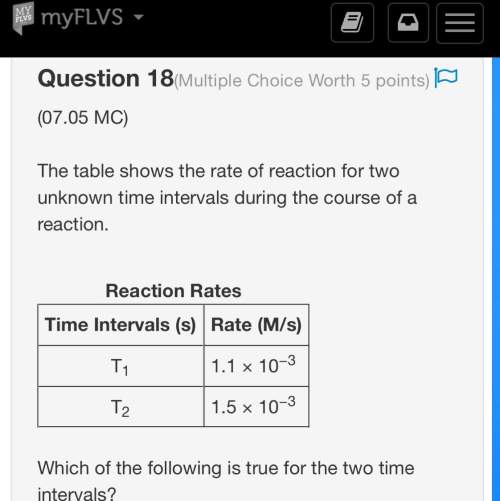
*worth 30 points + brainliest * the table shows the rate of reaction for two unknown time intervals during the course of a reaction. reaction rates time intervals (s) rate (m/s) t1 1.1 × 10−3 t2 1.5 × 10−3 which of the following is true for the two time intervals? a. t1 is longer than t2, and the concentration of reactants at the end of t1 is lower than that of t2. b. t1 is longer than t2, and the concentration of products the end of t1 is lower than that of t2. c. t1 is shorter than t2, and the concentration of reactants at the end of t1 is higher than that of t2. d. t1 is shorter than t2, and the concentration of products at the end of t1 is higher than that of t2.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1


Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
*worth 30 points + brainliest * the table shows the rate of reaction for two unknown time intervals...
Questions


Physics, 05.05.2021 09:30


Social Studies, 05.05.2021 09:30


History, 05.05.2021 09:30

Mathematics, 05.05.2021 09:30


Biology, 05.05.2021 09:30

History, 05.05.2021 09:30

Physics, 05.05.2021 09:30

Mathematics, 05.05.2021 09:30

Mathematics, 05.05.2021 09:30



Mathematics, 05.05.2021 09:30

Mathematics, 05.05.2021 09:30

Physics, 05.05.2021 09:30




