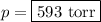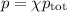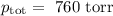Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 14:30
Which statement best identifies the process shown? the process must be fusion because energy is released. a.the process must be fusion because a heavier nucleus forms from smaller nuclei. b.the process must be fission because a large nucleus breaks into smaller nuclei. c.the process must be fission because neutrons are formed.
Answers: 1
You know the right answer?
The mole fraction of nitrogen in the air is 0.7808. this means that 78.08% of the molecules in the a...
Questions

Biology, 04.09.2020 19:01

Mathematics, 04.09.2020 19:01

Mathematics, 04.09.2020 19:01

History, 04.09.2020 19:01

History, 04.09.2020 19:01


Medicine, 04.09.2020 19:01


Mathematics, 04.09.2020 19:01

History, 04.09.2020 19:01

History, 04.09.2020 19:01


Mathematics, 04.09.2020 19:01




Mathematics, 04.09.2020 19:01

English, 04.09.2020 19:01

Mathematics, 04.09.2020 19:01

English, 04.09.2020 19:01