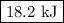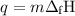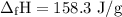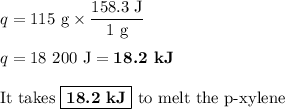Chemistry, 29.06.2019 05:00 lordcaos066
P-xylene, c8h10, has an enthalpy of fusion of 158.3 j g-1 and its melting point temperature is 13.2°c. how much heat is required to transform 115 g of solid p-xylene at 13.2°c into liquid p-xylene, also at 13.2°c?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
P-xylene, c8h10, has an enthalpy of fusion of 158.3 j g-1 and its melting point temperature is 13.2°...
Questions



Mathematics, 17.01.2021 01:00

Mathematics, 17.01.2021 01:00


Mathematics, 17.01.2021 01:00

History, 17.01.2021 01:00

Mathematics, 17.01.2021 01:00


Mathematics, 17.01.2021 01:00





Mathematics, 17.01.2021 01:00



Chemistry, 17.01.2021 01:00


Mathematics, 17.01.2021 01:00