
Chemistry, 28.06.2019 03:00 aidentrooper8629
During an experiment, 95 grams of calcium carbonate reacted with an excess amount of hydrochloric acid. if the percent yield of the reaction was 82.15%, what was the actual amount of calcium chloride formed? caco3 + hcl → cacl2 + co2 + h2o 105.3 grams 101.1 grams 95.6 grams 86.5 grams

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
During an experiment, 95 grams of calcium carbonate reacted with an excess amount of hydrochloric ac...
Questions



English, 11.06.2021 08:30

Social Studies, 11.06.2021 08:30

Biology, 11.06.2021 08:30



Chemistry, 11.06.2021 08:30

Mathematics, 11.06.2021 08:30

English, 11.06.2021 08:30

Mathematics, 11.06.2021 08:30

Biology, 11.06.2021 08:40


Chemistry, 11.06.2021 08:40



Mathematics, 11.06.2021 08:40

Physics, 11.06.2021 08:40


Mathematics, 11.06.2021 08:40

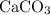 ?
?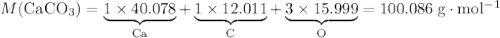 .
.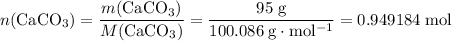 .
.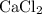 will be produced?
will be produced?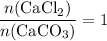 .
.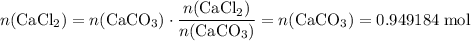 .
. of
of 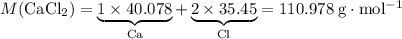 .
.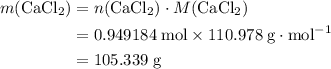 .
. .
. .
.

