
What happens to the molarity of a salt solution if the number of moles of salt in the solution is multiplied by three and the number of liters of the solution is also multiplied by three? the molarity of the solution remains unchanged. the molarity of the solution becomes three times as much. the molarity of the solution becomes six times as much. the molarity of the solution decreases.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
What happens to the molarity of a salt solution if the number of moles of salt in the solution is mu...
Questions


Mathematics, 18.02.2021 19:50


Mathematics, 18.02.2021 19:50

Mathematics, 18.02.2021 19:50

Arts, 18.02.2021 19:50



Mathematics, 18.02.2021 19:50

Mathematics, 18.02.2021 19:50


Mathematics, 18.02.2021 19:50








Mathematics, 18.02.2021 19:50

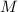 of a solution:
of a solution: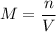 ,
, 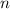 is the number of moles of solute in this solution, and
is the number of moles of solute in this solution, and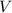 is the volume of the solution.
is the volume of the solution.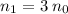 , and
, and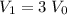 .
.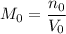 .
.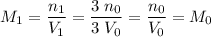 .
.

